People with Irritable Bowel Syndrome (IBS) often show differences in certain probiotic gut bacteria. Now, new research has illustrated how a heat-inactivated probiotic can offer IBS relief, gaining attention as a potential solution and IBS treatment.
Irritable Bowel Syndrome (IBS) is a complex and rather painful digestive disease. Its reputation circles around a lot of confusion and frustration: from medical professionals, patients, and scientists alike! This stems a lot from the great variety of patient experiences: IBS triggers are very individualized. Still, researchers are gathering more and more evidence that changes in the composition of the gut microbiome may be the common ground.
Quick recap: Probiotics are microorganisms, such as bacteria, that we can take orally to protect us from pathogens, keep our intestines intact and boost the production of beneficial nutrients. They are live microbes, and you’ll find most supplement options in-store feature various species of Bifidobacteria and Lactobacillus.
What Is IBS?
Overall, IBS is characterized by according to chief complaints: constipation (IBS-C,) diarrhea (IBS-D,) a combination of the two (IBS-M) or unclassified (IBS-U.) Generally, other symptoms, such as abdominal pain and bloating, can also come into the mix – and is commonly seen for people with IBS-C.
What all people with IBS have in common – regardless of their primary characterization – is that their symptoms match Rome criteria for IBS. This is a checklist used by medical doctors to detect IBS. Namely, this means they are checking whether your current condition is one when there is a “recurrent abdominal pain or discomfort and a marked change in bowel habit for at least six months, with symptoms experienced on at least three days of at least three months.”
On top of this, at least two of these three statements need to be true in order to be considered an IBS patient:
- Onset of pain is related to a change in stool frequency.
- Onset of pain is related to a change in the appearance of stool.
- Pain is relieved by a bowel movement.
What makes the IBS so frustrating, for medical doctors and patients alike, is that it often presents in drastically different forms amongst sufferers. There is no string of symptoms or clear triggers, that will cover the nearly billion people affected globally.
What Causes IBS?
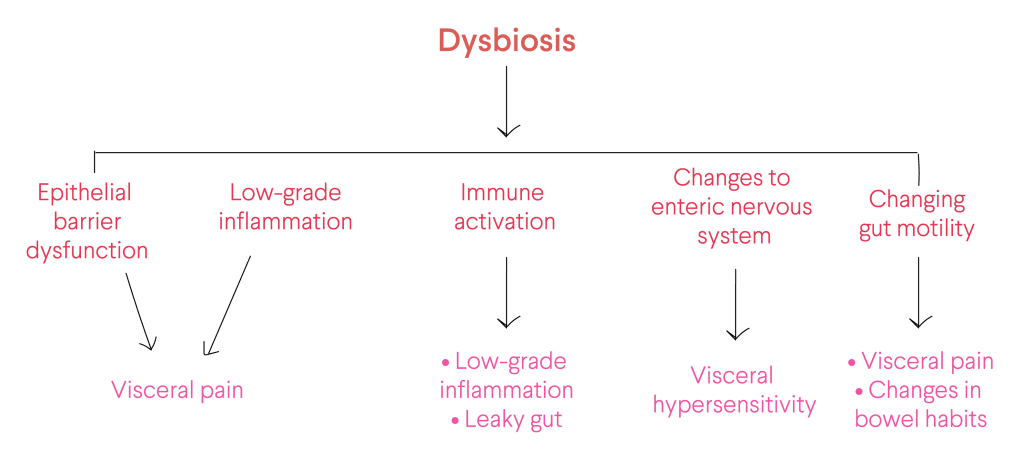
Unfortunately, there is currently no known cause – or cure – for IBS that scientists or medical doctors can say with enough certainty. Still, the internal gut lining of patients with symptoms is thought to be more permeable. Furthermore, scientists have proposed other additional mechanisms that seem to be involved in the pathogenesis of IBS, including alterations in the gut-brain axis, visceral hypersensitivity, immune system activation, low-grade inflammation and dysbiosis in the gut microbiota1.
Overall, current IBS therapy relies on lifestyle and symptom management. This means keeping track of specific triggers.
Typically, the most common IBS triggers are:
- Food. Many people report their IBS symptoms to worsen when they eat certain foods or drink certain beverages. The most common culprits tend to be wheat, dairy products, milk, beans, and carbonated beverages. Still, it’s important to note the role of food allergies or intolerance in IBS isn’t fully understood. True food allergies are rarely indicated to cause IBS.
- Stress. IBS often goes hand-in-hand with a powerful psychological component. Most people with IBS tend to experience worse or more frequent signs and symptoms when they are under greater stress. But, even though stress may aggravate symptoms, it doesn’t cause them.
- Hormones. Women are much more likely to have IBS, suggesting that hormones may play a role. Many women report that their symptoms amplify around their menstrual periods.
Ultimately, with so many avenues for IBS to individually arise, there are also a multitude of ways to treat IBS. These include various changes to your diet and lifestyle – hopefully, conducted in a personalised manner (where microbiome testing can often help).
The Link Between Your Microbiome and IBS
Ever-increasing research activity from the last decade has built up notable evidence that disruption in the diversity, richness, and composition of the gut microbiota – otherwise known as dysbiosis – plays a critical role in IBS pathogenesis2. Several mechanisms can lead to dysbiosis: overgrowth or depletion of specific species of bacteria, to alterations in bacterial richness3.
In IBS patients, gut dysbiosis has been linked with:
- Changing gut motility.
- Low-grade chronic inflammation.
- Raised pain perceptions and enhanced mucosal permeability, can eventually interfere with gut immunity and thus promote gut inflammation.
- Alterations of the enteric nervous system, as well as brain functions4,5,6.
Meanwhile, research aimed at mapping a microbiome signature of IBS has not been conclusive. Nevertheless, data does suggest richness in pro-inflammatory bacteria, and depletion of certain so-called good gut species has been noted in IBS patients7,8. In our own research, we have found certain microbes have been strong scientific links to IBS. Namely, people with IBS often report lower levels of Bifidobacteria, Collinsella aerofaciens, Coprococcus comes, Eubacterium rectale, Faecalibacterium prausnitzii, and Lactobacillus9. What’s more, they can often have elevated levels of Escherichia coli and Methanobrevibacter smithii.
People with IBS often report lower levels of: Bifidobacteria, Collinsella aerofaciens, Coprococcus comes, Eubacterium rectale, Faecalibacterium prausnitzii and Lactobacillus.
Moreover, a study looking at stool samples of 60 patients and 20 healthy controls revealed major variability in the microbiota of people with different IBS subtypes (IBS-D, IBS-C, or IBS-M type)10.
Other studies have demonstrated that therapeutically modifying the microbiota – such as through prebiotics, probiotics or fecal microbial transplantation – have been linked to improvements in IBS symptoms11,12,13. Taken together, it seems research really does add up, connecting the dots on a link between the microbiota and IBS. No wonder microbiome modification strategies are increasingly becoming an attractive option for IBS management. Now, a new study has found that one particular probiotic option has tremendous potential.
Using Dead Bacteria to Modify the Microbiome for IBS Relief
Scientists have recently reported that a heat-inactivated version of the probiotic bacteria Bifidobacterium bifidum can help relieve IBS symptoms14. The strain, B. bifidum MIMBb75, is one of a few select probiotics with proven efficacy in IBS.
The study, published in April 2020, researched randomised 443 people with IBS to receive either 2 capsules of the B. bifidum treatment or placebo. After 8 weeks, the results showed improvement in IBS-related pain, as well as overall symptom relief. Overall, 34% of participants who received the probiotic reported relief from stomach pain and overall IBS symptoms, compared with 19% of those taking the placebo. Even if there was a strong placebo effect – common in IBS studies – the probiotic treatment still did better than the placebo.
The probiotic group reported 1.7X greater treatment success.
Furthermore, patients who received the inactivated probiotic were shown to be significantly more likely to report satisfactory symptom relief, compared to placebo. Overall, the probiotic group reported 1.7X greater treatment success.
The team think these dead bacterial cells are somehow able to stick to the stomach lining in the same way live probiotics do. This may then strengthen the gut barrier, offering greater protection against bad bacteria and toxins, which could otherwise contribute to symptoms of IBS.
Notably, B. bifidum MIMBb75 had mucosal adhesive properties, adding to other suggestions that impaired intestinal barriers are perhaps at the root of IBS. The team thinks these dead bacterial cells are somehow able to stick to the stomach lining in the same way live probiotics do. This may then strengthen the gut barrier, offering greater protection against bad bacteria and toxins, which could otherwise contribute to symptoms of IBS.
For the first time, these findings suggest dead probiotics could be just as effective as live probiotics – opening up the chance for longer shelf life. Given the probiotic treatment worked in a third of IBS patients, it could spell a major shift in how we use microbes for bettering IBS.
Tips for Managing Your IBS
- Keep a Food and Symptom Journal. This will help you notice patterns in your flare-ups and help identify (or dismiss!) any IBS triggers.
- Avoid Inflammatory Foods. Managing your diet and lifestyle is probably the most effective and common way to support your symptoms. We’re only as healthy as the food we digest. Thus, it’s important to pay attention to what we eat – specifically when it comes to foods that no one can claim to be “health-generating!” In other words, stay away from sugar, refined flours, processed products, and the like.
- Have a Handy Toolbox. Certain herbs and supplements complement your lifestyle changes and help improve daily IBS discomfort. Namely, peppermint is well-known for its therapeutic properties – helping to increase gut motility and reduce abdominal cramping. Furthermore, ground flaxseeds can have laxative effects, and help get your symptoms moving. In this way, do your own research, and find what may aid your specific struggle.
- Keep Your Mental Game Strong. Stress and IBS are deeply intertwined. Thus, make sure to take care of your mental health: manage anxiety and stress, embrace meditation and long walks, or anything else that relaxes you. It’s truly worth it!
Remember, IBS manifests in a very individualised manner: what works for someone else might not work for you. Thus, taking a microscopic look at what’s actually going on in your body can be tremendously valuable. No wonder, we’ve crafted a dietary intervention program that caters just to that.